Water In Oil Emulsion Examples Cosmetics

Additives and ingredients like these from making cosmetics are perfect for cosmetic products that involve water in oil emulsion.
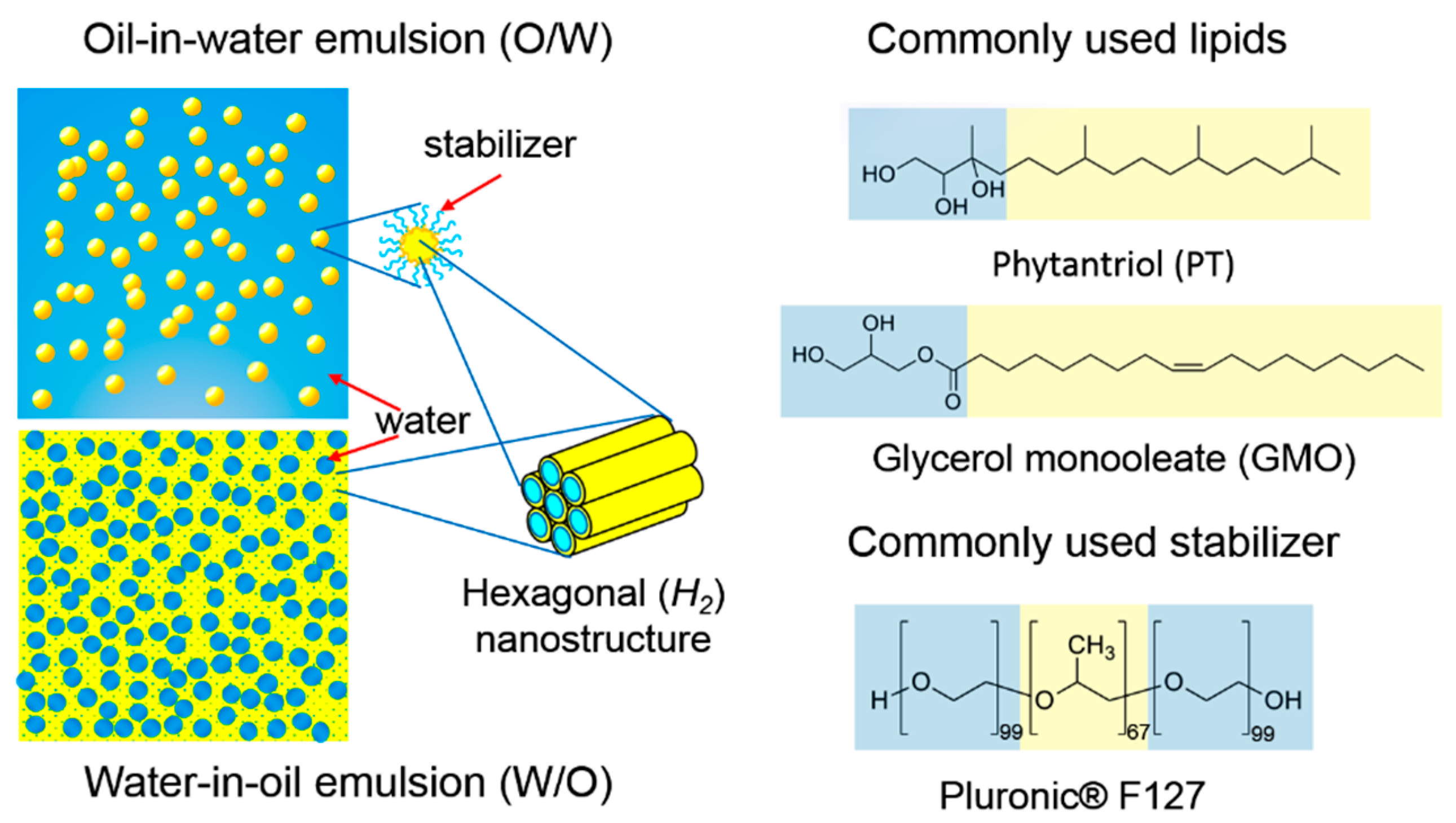
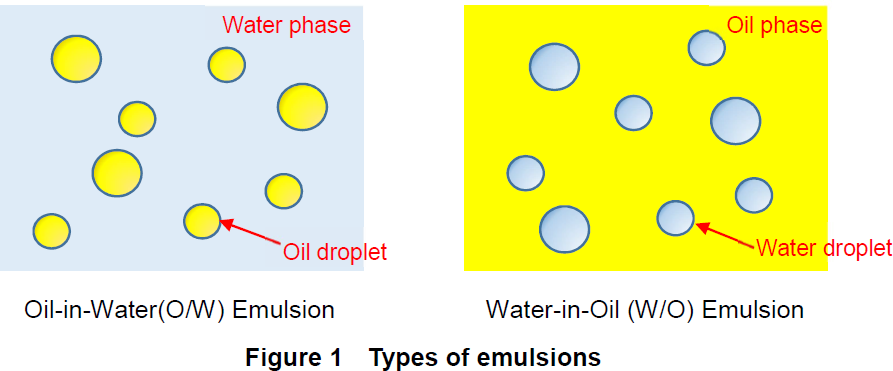
Water in oil emulsion examples cosmetics. Examples of emulsions. Simple emulsions are either oil suspended in a water phase o w or water suspended in oil w o. These emulsifiers are more soluble in oil than in water. Oil in water emulsions used in moisturizing products and food products such as milk mayonnaise and vinaigrette o w emulsions contain a low oil concentration.
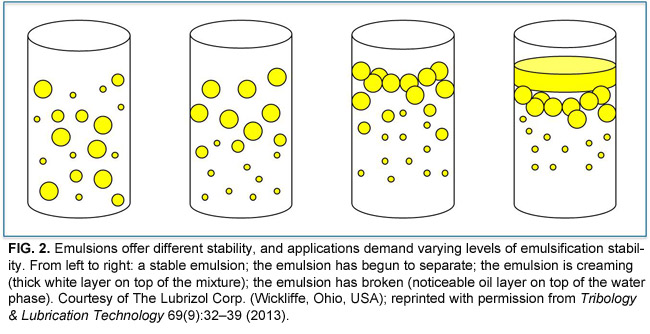
In terms of skincare common emulsifiers include polysorbate 60 cetearyl alcohol and glyceryl stearate any of which can commonly be found listed as ingredients in an emulsion most emulsions are formulated as oil suspended in water which is generally more stable and has less chance of separating than one made of water suspended in oil. They are mixable with water non greasy non occlusive and will absorb water. The emulsifier we choose determines whether the emulsion will be o w or w o. Thus there is a greater likelihood of coalescence.
O w emulsifiers keep oil drops packed in water. In addition to a reliable emulsifier it is recommended that you also use stabilizers thickeners in your formula to enhance the stability of the emulsion. Oil in water o w emulsifiers keep oil drops packed in water while water in oil w o emulsifiers keep water drops packed in oil. Cetearyl wheat straw glucosides and cetearyl alcohol.
Egg yolk is an emulsion containing the emulsifying agent lecithin. The dispersion medium in these emulsions is water. Since water and oil do not mix but stay separated an additional agent emulsifier is necessary to form a homogenous mixture keeping water and oil together. The oil comes in contact with skin first providing more greasiness.
Water in oil emulsifiers water in oil emulsifiers help in producing water in oil w o emulsions. Under certain circumstances oil and water can be mixed to a homogenous and stable solution called emulsion. Everyday examples of emulsions are butter milk and mayonnaise which all consist of oil and water. Formulating water in oil emulsions is inherently more difficult than oil inwater emulsions.
While there are several reasons for this probably the most important one is that these emulsions usually do not have a clearly defi ned electrical double layer surrounding the emulsion droplets. Oil and water mixtures are emulsions when shaken together. In w o emulsions water droplets are dispersed in oil oil encases water. An emulsion is a stabilized mixture dispersion of two or more immiscible liquids like oil and water.
For example you can add 0 2 0 5 xanthan gum to the water phase and or 2 cetyl alcohol to the oil phase. They have hlb between 2 5 6 are non ionic or polymeric. Most emulsions we make are o w which are generally more stable and easier to formulate and this is the type of emulsion we will be discussing in this article. There are 2 types of emulsifiers.