Water Distillation Unit Principle

So water can be found as ice water and.
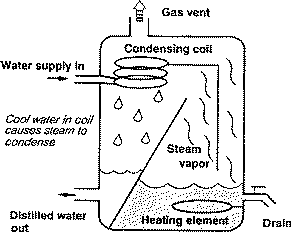
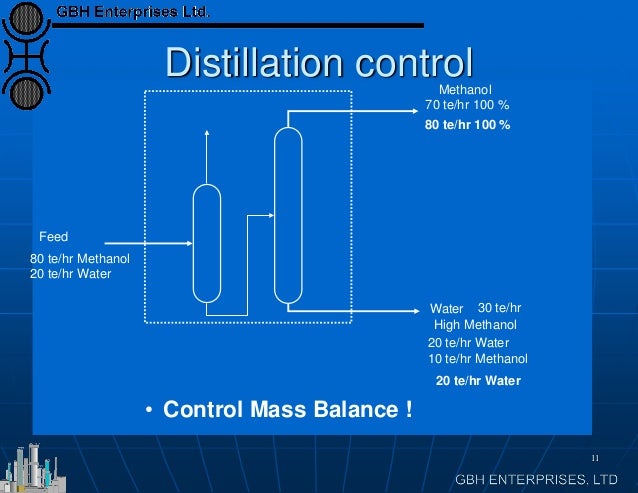
Water distillation unit principle. The number of effects is inversely proportional to the kw h m 3 of water recovered figure and refers to the volume of water recovered per unit of energy compared with single effect distillation. Water distillation unit metal table model water distillers produce highly treated and disinfected water for laboratory usage. Laboratory distillation operation and principle of the new distillation unit distillation is a thermodynamic separation process that utilizes different boiling points of the components in the mixed liquid or liquid solid system to evaporate the low boiling components and then condense to separate the entire component. Water is heated to boiling in an enclosed container.
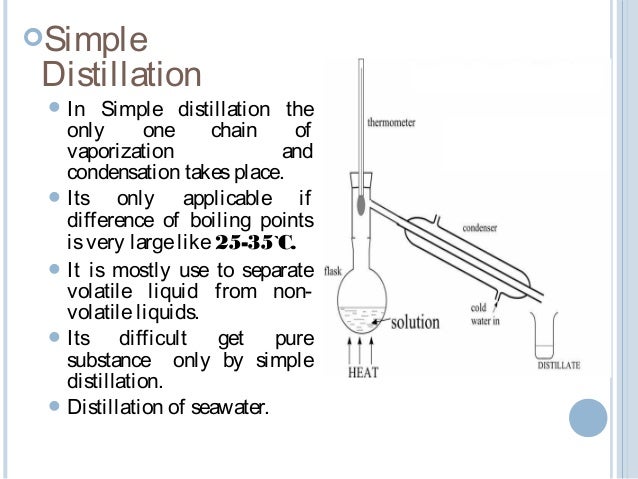
Water distillation principles every element can exist in three states. As a liquid as a solid and as a vapor which mostly depend on it s temperature. Distillation is based on the fact that the vapour of a boiling mixture will be richer in the. To separate a mixture of liquids the liquid can be heated to force components which have different boiling points into the gas phase.
Nature uses a form of distillation to turn salt water seawater into fresh water rain. It involves the separation of a mixture based on the difference in the boiling point or volatility of its components. Principles of distillation the principle for operation of a distiller is simple. The reason for the wide acceptance of distillation is that from both kinetic and thermodynamic points of view distillation offers advantages over other existing processes for.
Principles control troubleshooting definition of distillation a process in which a liquid or vapourmixture of two or more substances is separated into its component fractions of desired purity by the application and removal of heat. This applies to water too. As the water evaporates inorganic chemicals large non volatile organic chemicals and microorganisms are left behind or killed off in the boiling chamber. Why do you use distillation to recycle waste solvents.
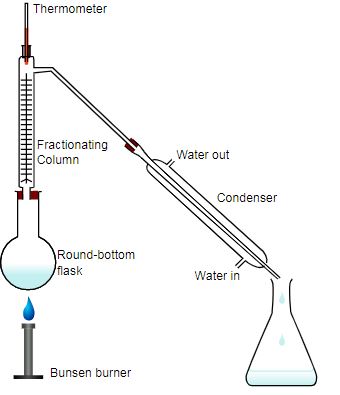
Distillation definition distillation is a widely used method for separating mixtures based on differences in the conditions required to change the phase of components of the mixture. Simply distillation is the process in which a liquid is vaporized turned to steam recondensed turned back into a liquid and collected in a container. The mayor differences between the bath b 491 and b 495 are the bath size and temperature range. The distillation process removes minerals and microbiological contaminants and can reduce levels of chemical contaminants.
Distillation is the oldest separation process and the most widely used unit operation in industry. The b 495 is additionally equipped with a automatic water level control bath replenisment.